Large-scale screening rapid antigen tests campaigns among the general population may seem to be a promising technique for guiding containment efforts, however the difficulties associated with such a strategy should not be underestimated, and the success of such campaigns remains in doubt.
First and foremost, testing millions of individuals per week, along with all of the necessary pre-and post-analytical work, is a time-consuming and labor-intensive process. For the second time, rapid antigen tests are the only viable choice at this time in terms of capacity and cost for testing on a large scale, such as millions of individuals. lean more about rapid antigen test commonly called RAT at https://clinicalsupplies.com.au/collections/rapid-antigen-tests
However, given their existing lower performance, as has been stated above, there are difficulties. However, even if the performance of rapid antigen tests continues to improve over time, the issue of low prevalence in the general population and, therefore, the large number of false positives will continue to be a concern.
In other words, a significant number (perhaps as much as more than 50%) of all “positive” rapid antigen tests will be false positives in reality. If persons with a positive test result are expected to isolate themselves, as would be required for the technique to be successful, this has the potential to undermine their acceptance of the rapid antigen tests.
This is tough to manage at a large scale. Because of this, many individuals would have limitations in their everyday life, including their capacity to work, even if they did not have the virus in their system. If such a scenario is possible, policymakers must assess if there is enough public support for it to be implemented successfully. Click here to read some good tips that you can try at home while performing rapid antigen tests.
Mass screening is only successful if individuals are willing to be tested and if those who are recognized as positive isolate swiftly. People may be reluctant to isolate if they have doubts about the authenticity of the test findings, which is particularly true if they believe the results are bogus.
A number of nations are now experimenting with population-wide screening, but the results have been inconsistent so far, and the projects are proving difficult and expensive to implement. From a technical aspect, next-generation sequencing (NGS) has the potential to be an acceptable option in the future, depending on the circumstances.
However, this rapid antigen tests technology is still in the early stages of development. NGS is a highly sensitive and specific test modality that has the capability of delivering extraordinarily high throughput rates at extremely low costs. Some firms and labs are expanding COVID 19 testing capacity via the use of next-generation sequencing (NGS), which can test up to 10,000 samples at a time with a turnaround time of 24 to 48 hours for findings.

What should I do if the COVID-19 rapid antigen tests results are negative?
In humans, the COVID-19 rapid antigen tests are a rapid membrane-based lateral flow immunoassay that can be used to identify SARS-CoV-2 antigens in nasopharyngeal swab specimens in less than an hour. It is possible to identify the presence of SARS-CoV-2 viral antigens in human bodies with the use of a COVID-19 rapid antigen tests, which may then be used to initiate preventive isolation measures in order to minimize the spread of the virus. A person who is at or near the peak of infection may be identified using one of these rapid antigen tests. So the issue becomes, how should I interpret the results of the antigen quick test?
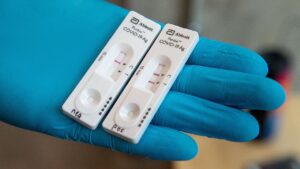
Structure of the COVID-19 rapid antigen tests Cassette
The antigen test cassette for COVID-19 is coated with two lines of antigen (the control line and the test line). A colored line will appear on the test strip if the specimen has SARS-CoV-2 antigens, suggesting that the specimen contains SARS-CoV-2 antibodies coated on the (T) Test line area and that the specimen contains SARS-CoV-2 antigens.
A negative result is shown by the absence of a colored line in the (T) Test line area, which indicates that the antigens were not present in the material. It is always possible to see a colored line in the (C) Control line section, which indicates that the test method was followed correctly and that the test components worked as planned.
Results of the Valid rapid antigen tests
Two colorful lines emerge as a result of a successful test.
It is essential that a colored line appears in the Control (C) section at all times, and that another line appears in the Test (T). Antigens for SARS-CoV-2 have been detected in the sample, resulting in a positive result.
When the instructions are carefully followed, a positive antigen test result is considered accurate; however, there is an increased chance of false-negative results — meaning it is possible to be infected with the virus while having a negative result — when the instructions are not carefully followed.
A colored line emerges in the Control (C) zone as a result of a negative result.
In the Test (T) area, there is no line to be seen.
In order for the test to be legitimate, a colored line should always appear in the Control (C) zone, just as it does in the case of a positive result. A negative result indicates that the detection threshold for SARS-CoV-2 antigens in the sample has not been met in this particular case. Even if the quantity of antigens in the sample is too low to be identified by the rapid antigen tests, there is still a risk of infection developing in the sample.
Don’t use these results in the absence of the control line.
Generally speaking, insufficient diluent volume or inappropriate procedural procedures are the most frequent causes of the disappearance of the control line in the sample. Review the protocol and do the test with a different instrument if necessary.
Rapid antigen tests are generally affordable, and the majority of them may be performed right at the patient’s bedside. The majority of the rapid antigen tests that are now permitted produce findings in around 15 minutes. When it comes to identifying the presence of viral nucleic acid, antigen testing for SARS-CoV-2 are often less sensitive than real-time reverse transcription polymerase chain reaction (RT-PCR) and other nucleic acid amplification rapid antigen tests (NAATs). RT-PCR, on the other hand, may detect amounts of viral nucleic acid that cannot be grown, indicating that the presence of viral nucleic acid does not necessarily signal the existence of contagiousness.
Please make a decision on the results of gotten from the rapid antigen results within 15-30 minutes of receiving them. If you check again in a few hours, the result will no longer be valid.